There is consensus among stakeholders that patient engagement at different points of the medicines lifecycle is critical to fostering patient access to innovative therapeutic solutions, and delivering better health outcomes for patients. IMI PARADIGM, a public private consortium consisting of patients, academia, industry, HTA bodies and regulators, developed a toolkit to make patient engagement in medicines development easier for all. The toolkit has very practical tools that can be used as such or localised to reflect institutional specificities:
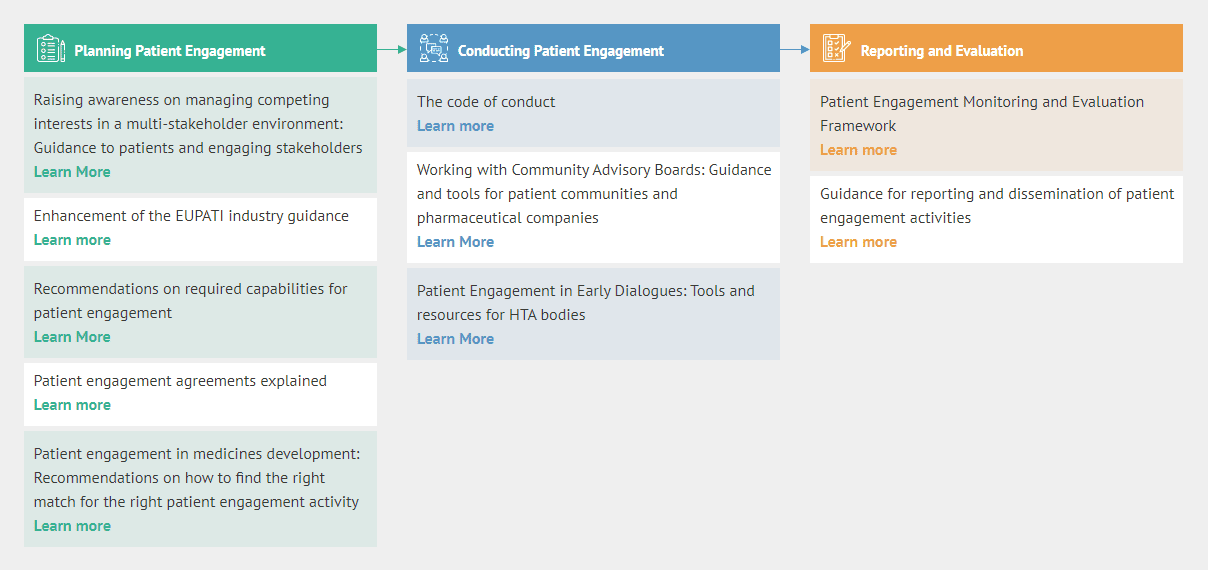
Planning Patient Engagement
- Raising awareness on managing competing interests in a multi-stakeholder environment: Guidance to patients and engaging stakeholders
- Enhancement of the EUPATI industry guidance
- Recommendations on required capabilities for patient engagement
- Patient engagement agreements explained
- Patient engagement in medicines development: Recommendations on how to find the right match for the right patient engagement activity
Conducting Patient Engagement
- The code of conduct
- Working with Community Advisory Boards: Guidance and tools for patient communities and pharmaceutical companies
- Patient Engagement in Early Dialogues: Tools and resources for HTA bodies
Reporting and Evaluation
- Patient Engagement Monitoring and Evaluation Framework
- Guidance for reporting and dissemination of patient engagement activities
To tools are available free of charge at the following link: http://imi-paradigm.eu/petoolbox/
You’re invited to test it, localise it and disseminate the information in your organisation and to your partners.