On 16 March 2021, on the occasion of Brain Awareness Week 2021, the European Brain Council, in partnership with the European Federation of Neurological Associations & GAMIAN-Europe, held an event on Patient Engagement in EU-Funded Brain Research Projects.
The event aimed to shed light on the current state of patient engagement in EU-funded brain research projects, exploring how patients have been involved to date, their experiences in this involvement, the challenges continued to be faced, examples of patient-involved projects and initiatives and looking at what can be done to improve engagement.
EBC, EFNA and GAMIAN-Europe were pleased to welcome over 100 participants. The event was opened by Prof Monica Di Luca, President of EBC, who – speaking as a basic scientist – highlighted the fundamental importance of patient involvement in research in order to gain knowledge from the lived experience of patients to boost research findings to work towards understanding and discovering proper treatment and cures for brain disorders. Patient engagement is a central force in many EBC activities, particularly in the European Brain Research Area (EBRA) project and in the EBC Policy Roadmap ‘Brain Health in Europe: Fostering Innovation, Improving Outcomes’ released on the day.
From here the programme shifted to setting the scene, with Irene Norstedt, Director, People Directorate, DG Research and Innovation, European Commission; Erik Van der Eycken, EU Research Project Officer, GAMIAN-Europe presenting their views on the challenges of engaging patients in research; and Joke Jaarsma, President of European Federation of Neurological Associations (EFNA) President & Treasurer of EBC.
One of the key issues to put on the table is the understanding of the role and expectation [for patients in research]. It is very clear that it has to be a real participation and not a token participation. This is something that we’ve had for many years in the European Commission with projects often having a requirement to have patient participation in research that we are funding and what we really need to highlight is the importance to make sure that patients and/or patient representatives are not put there as tokens but really are given an active role.
Communication is a very important aspect and key challenge in patient involvement. Overcoming language barriers is not only understanding the various European languages but also the type of language that you have between the researchers and the patients/patient representatives. In my experience, in different projects we talk a very different language. This is not only between patients and researchers but also between patients and patient representatives, making sure we understand how to communicate their wishes, their requirements.
How to actually involve patients in the research process is still not fully defined. In spite of the fact that patient involvement has become a key element in patient-centred healthcare and it is clear that patient involvement such as working with the clinical research community as co-creators of clinical trials can help make research outcomes and end points more meaningful to both patients and caregivers. There is enough evidence by now that this can help make research more focused and acceptable, starting with asking the right research questions, creating appropriate clinical trials right through to regulatory HTA.

After outlining the ongoing challenges, both from the funder perspective and the patients’ perspective, three invited speakers presented work within their organisations and/or projects, giving concrete examples of actively engaging patients: Pierre Meulien, Executive Director of the Innovative Medicines Initiative (IMI); Paola Zaratin, Project Coordinator of MULTI-ACT; and Patrik Vankrunkelsven, Director of the Belgian Centre for Evidence-based Medicine (CEBAM).
European initiatives such as the Innovative Medicines Initiative (IMI) have spearheaded the growth of involving patients in European research, encouraging patient involvement in their activities and project, and involving patients as speakers and panelists in their events and consultations. IMI, as a funding body, needs to continuously work to innovate and update their strategic thinking, which saw the launch of the IMI Pool of Patient Experts last year. This works to “put the patients at the same level as any scientific, clinical, ethical or economics expert and being able to use their own patient experience as a key asset in each stage of the research project life cycle” and gathers patients from all across the EU that can be called upon to help provide guidance within IMI activities and projects. There are currently 157 patients/carers from 26 EU countries registered – the pool is also open to family members and carers. 30% currently cover neuropsychiatric and neurodegenerative disorders.
H2020-funded projects such as MULTI-ACT were launched with the aim to increase the impact of health research on people with brain diseases and to create and implement a new model allowing for the effective cooperation of all relevant stakeholders. The MULTI-ACT White Paper for innovative routes for patient engagement is a high-level policy-oriented document addressing key actions to be taken in the short, medium and long term by policymakers and research & innovation funders to foster patient engagement in health R&I. It calls for policymakers to take action and implement innovative routes for patient engagement in R&I, leading to research outcomes that matter most to patients and society.
One of the main activities of the Belgian Centre for Evidence-based Medicine (CEBAM) is validating guidelines, which has seen a very fast-paced evolution of the importance of patient involvement. “Just 5 years ago we judged patient involvement as ‘not so important’, however, nowadays, it is really required to involve patients in the development of guidelines, otherwise we cannot validate it.” CEBAM is currently working on the development of a website on “Health and Science” for patients, trying to augment health literacy with the understandable and easy-to-digest presentation of thousands of health guidelines alongside fact-checking of [research-related] news.
The event then shifted back to the original panel of Irene Norstedt, Director, People Directorate, DG Research and Innovation, European Commission; Erik Van der Eycken, EU Research Project Officer, GAMIAN-Europe presenting their views on the challenges of engaging patients in research; Joke Jaarsma, President of European Federation of Neurological Associations (EFNA) President & Treasurer of EBC, also joined by Tomislav Sokol, Member of the European Parliament (HR, EPP). MEP Sokol spoke on the need for structured dialogue and engagement of patients at the EU-wide level, citing the challenges of healthcare being a Member State competence but also the small steps towards change gained by EU-wide programmes/initiatives such as the Cancer Plan and EU4Health. The panel went on to react to the concrete solutions presented by IMI, MULTI-ACT and CEBAM and answered questions from the audience.
The fact that obviously primary competence of organising health care systems and regulating patients’ rights is on the national level; but this was in many cases just used as an alibi for lacking common EU vision of what kind of healthcare provision we want. This is definitely changing and I think that on EU level we have some positive developments, obviously the COVID-19 pandemic has put healthcare, patient needs and patient engagement more to the centre of EU decision making.
We need more structured dialogue and engagement with patient organisations, not just at a formal level in conferences, events, etc. but to really engage them in real decision making in working groups, in creating white papers and at the very start of different legislative and policy initiatives.
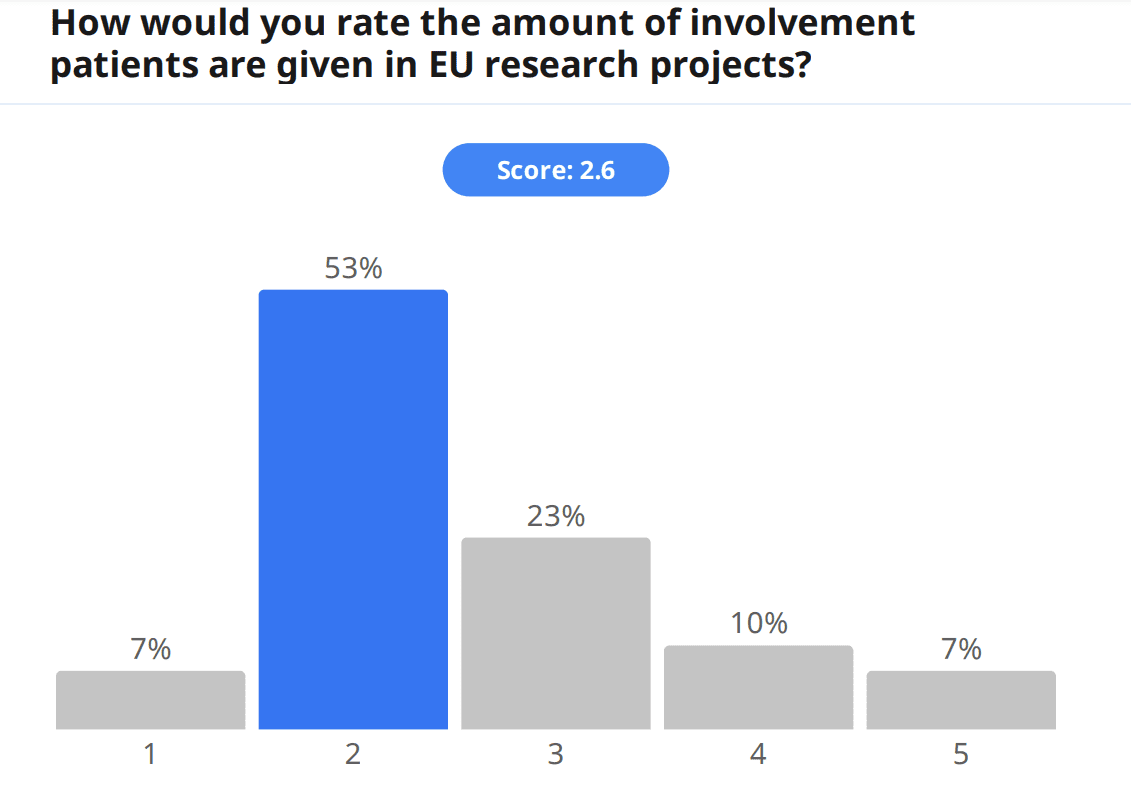
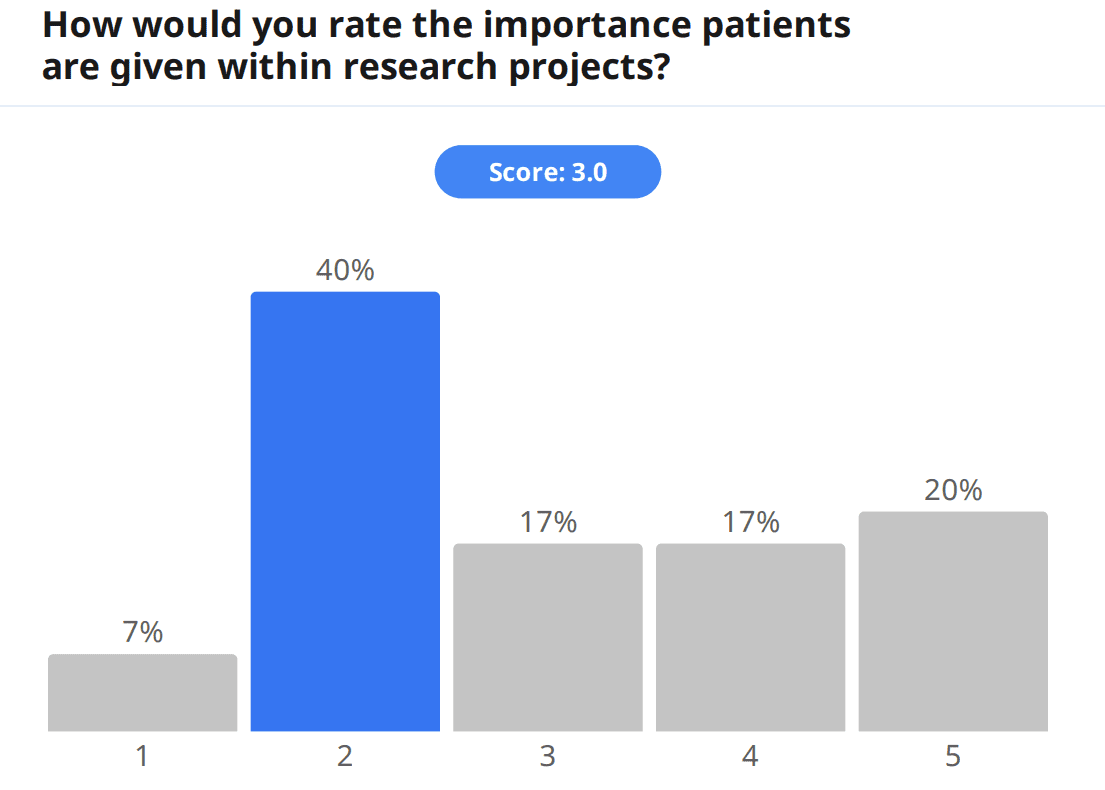
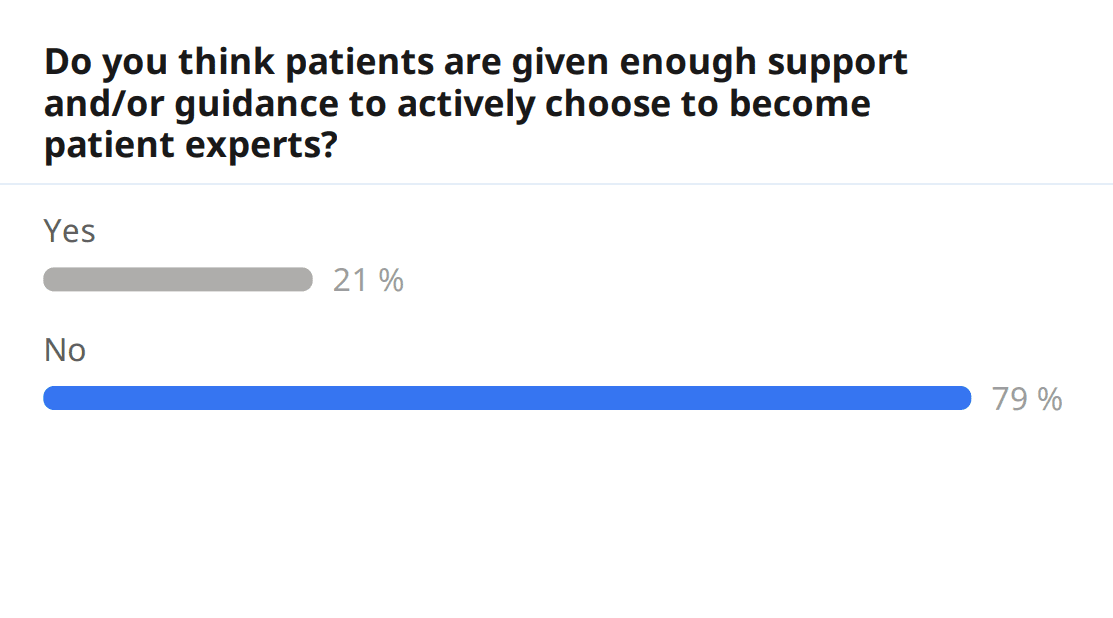
Apart from engaging amongst themselves in the event chat and with speakers during the Q&A, audience members were also able to engage directly in the topic by filling out a pre-event survey, which gathered information about attendees. Around a third of the audience filled out the survey, and of the surveyed audience members, 21% were patients. When asked to rate the amount of involvement patients are given in EU research projects, the audience gave a score of 2.6/5; furthermore, when asked to rate the importance patients are given within research projects, the score was also low, 3.0/5. Additionally, and on a point touched by speakers, the audience was also asked whether they felt patients are given enough support and/or guidance to actively choose to become patient experts, and 79% answered no. In fact, much more work is yet to be done for patient engagement.
The audience was also asked to provide input on the same question posed to the speakers: what are the challenges of engaging patients in research?
These were some responses received:
“Researchers are afraid of losing focus if they involve patients.”
“Many patients (& carers!) already have a hard time because they are dealing with a serious disease. And they don’t get financially rewarded for investing their time and energy. In patient organizations, there already is a lot of extra work to do: bureaucracy & supporting others takes almost all our energy and time already. It’s difficult to find committed, long-term volunteers.”
“The Idea that it’s too hard to involve patients.”
“Connecting patients with researchers, providing patients with adequate information on their diagnosis, treatment possibilities, and clinical trials.”
“Time to generate a diverse and representative patient pool: Involving only 1 patient in a research project leads very easily to overfitting, especially as the patient involved is usually not a good representation of the whole population. It is important to meet with several patients, also the invisible majority, to build solutions that make sense for the targeted group. Experience on how to involve patients (how to maximize their value to the project): Researchers sometimes have a tendency to patronize patients, or to project their views on the patients’ views. Researchers could learn how to interact with patients (and every patient can bring something different to the table) to maximize the value each patient can bring to the project.”
“PPI groups are often not fully representative of the total patient population. They tend to be better informed and higher educated. How can we Ensure that the views of the PPI group truly represent the wider patient population?”
“There is often quite a big gap between the results from research and clear, concrete messages of how that research benefits patients. Researchers prefer to stay close to their research findings, that are limited to certain situations or conditions and don’t want to oversell their research. Patients on the other hand want clear and simple answers and solutions. In addition, researchers often want to understand how something works (i.e. the genetics of a disease) while patients want to know how they can get better. It is often difficult to explain why research is such a slow process, and that it can take many many years before fundamental research is translated into clinical care.”
“[Patients] not knowing about opportunities, what to expect, thinking that they need to have technical knowledge or be highly educated, sub-groups in particular not hearing about it or not feeling things apply to them or that they their views would be valued etc.”
“Patients should be introduced to PPI in a pragmatic, practice-oriented way; have access to support, should have ongoing feedback.”
“Do patients need to be involved in all types of research? Does all research need to be relevant for patients? A lot of research is done to gain knowledge on a topic that seems not relevant for patients now but which will benefit patients and society in the long term. We cannot only focus on short term goals in research projects. That’s not how science works. The biggest challenge is to define the research domains where patients can directly contribute and use their subjective experience. It makes no sense to involve patients in research in which they cannot use their experience. Their subjective experience is the most valuable expertise they have but patients do not have to become neuroscientists themselves.”
Lastly, together with our speakers, the question “how can patient involvement in research be improved?” was explored:
“Patients’ education and training so patients and researchers can understand each other, facilitate peer training (patients who have been involved in research training other patients who can be future research participants), make patient involvement in research more visible to the public, HTA bodies….”
“Generating patient pools and developing tools to generate fair involvement for all the parties.”
“Meaningful involvement of Patients in the research from the design of the research proposal, development, results through to the implementation and treated as full partners in research.”
“To have patients involved from the very beginning on a patient council where they are properly compensated as everyone else in the project. This would help to inform on the barriers and would encourage PwP to take part in the research project as it has been developed with an understanding of the lives experience. Also to include patients in what they want looked at not always what the pharmaceuticals want.”
“More financial support, especially for European disease-specific federations.”
“Creating patient councils to advise researchers on patient priorities, providing opportunities to bring patients and researchers together.”
“To the groundwork of meeting with patients, in their home setting, in their clinical setting, and learn to see the world through their lenses. – Accept that each patient is a human, with different interests, capacities, focuses, than the other patients/humans: learn how to maximize the value of each human to the research question.”
“Now, it is often researchers who involve PPI, but it can also be done the other way around, that PPI contact researchers if they see a problem that needs to be addressed.”
“Involving patient representatives already at the proposal writing stage, and have them as partners in consortia, is already a big step forward. The next step is to involve patients in formulating the research questions and to have a real dialogue between researchers and patients, at all stages of the research (i.e. citizen science).”
“By increasing awareness of researchers about PI and reaching out more actively to a more diverse group of patients.”
“Giving the sense of being and active actor, and giving the information to understand the data management principles and best practices.”
“By shared learning in groups consisting of both patients and researchers.”
“Patient involvement is a too broad and too general concept. Patient involvement can only be improved if both the patients and the researchers are aware of the value of involving patients. That’s why an effort should be put in explaining to basic scientists why they should involve patients in their research. Good arguments need to be provided but might be difficult to find for some areas in research. Only by seeing the added value/expertise/experience that patients bring, researchers will be convinced. For now, researchers involve patients because the commission or their institutions ask for it and their chances to be funded will increase. This is not a good motivation to include patients. In addition, researchers should become more aware of the importance and relevance of soft, “subjective”, phenomenological data instead of only focusing and trusting objective data (e.g., brain imaging) which are not reflecting the experience of the patients and are thus not relevant to patients. Researchers should be “obliged” to include such data in their research.”
“Make patients AND researchers aware of the advantages of patient involvement.”
The panel discussion was closed by moderator, Frédéric Destrebecq, Executive Director of the European Brain Council, echoing the importance many speakers placed on “driving a change” for patients and patient engagement in EU research.
Hilkka Kärkkäinen, President of GAMIAN-Europe, closed the event emphasising the importance of the discussion held on the day and the fact that it is clear that most patients and patient representatives still feel like not enough support is given to have their voices fully heard in research. “All stakeholders should know that patient involvement is meaningful and valuable and that patients are ready, willing and able to get involved. It is not acceptable to approach patients and/or patient organisations last minute looking for endorsement or help in disseminating research outcomes—they should be involved from the start!”
Hilkka highlighted the continued need for the establishment of patient expert pools, trust and relationship building amongst stakeholders and training of expert patients and researchers (much like EUPATI). She finished her address calling for EU policymakers to recognise the value of involving patients in research, including financial support for patient organisations to help foster grassroots patient engagement in research.
Thank you to all who attended and for your active engagement. The chat was very active during the event and we would like to highlight and share a few resources shared and discussed:
- IMI Patient Engagement Info
- IMI Project Factsheets
- Online e-learning platform aimed at increasing awareness of mental health hurdles that ensued after the COVID pandemic, along with some concrete strategies for patient engagement
- MULTI-ACT Project Outcomes
- EBC Policy Roadmap “Brain Health in Europe: Fostering Innovation, Improving Outcomes”